Signifier Medical Introduces New eXciteOSA® Product Update, Expanding Access to Sleep Apnea Therapy for More Medicare and Medicaid Patients
London, UK – March 6, 2025 – Signifier Medical Technologies Limited (“Signifier”), a global leader in medical technology for sleep-disordered breathing, today announced the introduction of its newest SKU, 18010, which features a hardware remote control specifically designed to meet Centers for Medicare & Medicaid Services (CMS) requirements for reimbursement. This major development ensures that eXciteOSA® therapy can be more readily accessed by Medicare and Medicaid patients, marking a significant step in Signifier’s mission to make root-cause sleep apnea solutions available to patients across the United States.
“eXciteOSA is a life-changing device helping patients address a root cause of sleep apnea and other sleep disorders. This milestone underscores our ongoing commitment to innovation, as well as our determination to expand treatment access through alignment with CMS requirements,” said Mujtaba Chohan, Head of Finance and Director at Signifier. “Today’s announcement is a testament to the collaborative efforts of our multidisciplinary teams, and I am proud of the progress we’ve made in ensuring that these therapies are available and affordable to as many people as possible.”
Key Benefits:
- Medicare and Medicaid Reimbursement: By introducing this hardware remote control, Signifier has met the criteria to classify its eXciteOSA® therapy under CMS HCPCS Codes (E0490 and E0491), a vital step in ensuring comprehensive coverage and reimbursement for eligible patients.
- Hardware Innovation: The remote control simplifies device usage for patients, offering a user-friendly interface that aligns with the durable medical equipment (DME) requirements set forth by Medicare and Medicaid.
- Consistent, Effective Treatment Experience: The new remote control maintains the same clinically proven efficacy and convenience that patients expect from eXciteOSA®, making therapy more accessible without altering its effectiveness.
Stay informed! Sign up here for exclusive updates on the new eXciteOSA® remote control’s availability and reimbursement coverage in your state.
Continuing Momentum
This development builds on Signifier’s recent momentum, including eXciteOSA® gaining Medicaid coverage to serve patients in Georgia and South Carolina. “The introduction of this new remote control allows us to reach more patients, through Medicare and Medicaid, who desperately need alternative therapies for their sleep apnea,” said Yasser Zayni, Head of Commercial, Clinical and Compliance at Signifier. “Sleep apnea affects over 50 million Americans, yet we are only now beginning to see real innovation when it comes to treatment. With this new SKU 18010, we’re building on that progress, ensuring Medicare and Medicaid patients across the country have access to this innovative, user-friendly therapy.”
About Signifier Medical Technologies
Signifier is a pioneer in addressing a root cause of sleep-disordered breathing. The Company is focused on developing and commercializing innovative and non-invasive solutions to help people breathe normally and naturally all night – without needing to use a wearable medical device or a surgical implant. Founded in 2015, Signifier is at the forefront of sleep therapy, with a mission to develop therapies to improve population health, increase the quality of patients’ healthcare experience, and generate healthcare savings. Signifier’s headquarters are in London (UK).
About eXciteOSA®
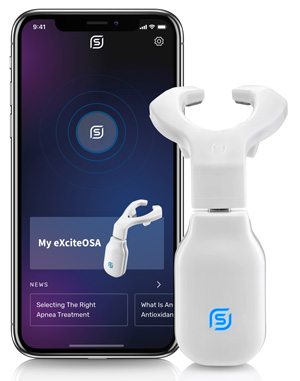
eXciteOSA® is the first FDA-authorized daytime therapy that treats a root cause of snoring and mild obstructive sleep apnea. Using gentle, non-invasive neuromuscular electrical stimulation (NMES), it strengthens the tongue muscles to prevent airway collapse during sleep. With just 20 minutes of daily daytime use, eXciteOSA® delivers clinically proven results, offering a comfortable, effective alternative to invasive nighttime therapies.
Signifier is dedicated to engaging with the sleep research community to produce high-quality evidence from rigorous clinical trials.
To learn more about eXciteOSA®, please visit www.signifiermedical.com or www.exciteosa.com.
Contacts
Media:
Jay Cranston
Signifier Medical Technologies LLC
[email protected]
Investors and Partners:
Investor Relations
Signifier Medical Technologies LLC
[email protected]



 The GOOD DESIGN award is presented by
The GOOD DESIGN award is presented by 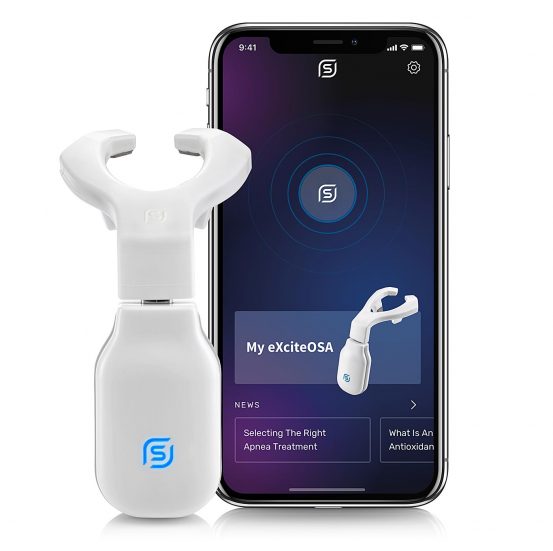


 Originally founded to recognize exceptional achievement in industrial design, the program has since grown with thousands of entries submitted by design teams across the globe, making IDEA one of the largest and most widely anticipated annual awards programs in the world.
Originally founded to recognize exceptional achievement in industrial design, the program has since grown with thousands of entries submitted by design teams across the globe, making IDEA one of the largest and most widely anticipated annual awards programs in the world.
 Mr. Hess is the former President & CEO of Bose Corporation, where he worked for 24 years across multiple leadership and operational roles. During his tenure, Mr. Hess was instrumental in Bose’s evolution from a speaker and DVD company to a leader in headsets and wireless products. Most recently, he was leading Bose into connected wearables and health products. He will be located in Boston, MA, where he will have responsibility for growing Signifier’s US infrastructure in preparation for the commercialisation of the company’s products.
Mr. Hess is the former President & CEO of Bose Corporation, where he worked for 24 years across multiple leadership and operational roles. During his tenure, Mr. Hess was instrumental in Bose’s evolution from a speaker and DVD company to a leader in headsets and wireless products. Most recently, he was leading Bose into connected wearables and health products. He will be located in Boston, MA, where he will have responsibility for growing Signifier’s US infrastructure in preparation for the commercialisation of the company’s products.